| CAS
NO. |
839-90-7 |
|
| EINECS
NO. |
212-660-9 |
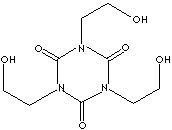
| FORMULA |
C9H15N3O6 |
| MOL
WT. |
261.23 |
|
H.S.
CODE
|
2933.69.8090 |
|
TOXICITY
|
|
| SYNONYMS |
1,3,5-Tris(2-hydroxyethyl)-1,3,5-triazine-2,4,6-trione;
THEIC; |
| Tris(hydroxyethyl) isocyanurate; Tris(beta-hydroxyethyl) isocyanurate;
N,N',N''-Tris(2-hydroxyethyl) isocyanurate; 1,3,5-Tris(2-hydroxyethyl)
isocyanurate; 1,3,5-Tris(2-hydroxyethyl) isocyanuric acid;
1,3,5-Tris(2-hydroxyethyl) cyanurate; Tris(hydroxyethyl) cyanurate;
Tris(2-hydroxyethyl) cyanurate; Tris(2-hydroxyethyl)-1,3,5- triazinetrione;
1,3,5-Tris(2'-hydroxyethyl)isocyanuric acid; |
| SMILES
|
n1(c(n(c(=O)n(c1=O)CCO)CCO)=O)CCO |
|
CLASSIFICATION
|
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
White crystalline powder |
| MELTING
POINT |
134
C - 137 C |
| BOILING
POINT |
|
| SPECIFIC
GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| pH |
|
| VAPOR
PRESSURE |
|
|
REFRACTIVE
INDEX
|
|
|
NFPA
RATINGS
|
|
|
AUTOIGNITION
|
430
C
|
| FLASH
POINT |
270
C
|
| STABILITY |
|
|
GENERAL
DESCRIPTION & APPLICATIONS
|
|
Triazine is the chemical species of six-membered heterocyclic ring compound with
three nitrogens replacing carbon-hydrogen units in the benzene ring structure.
The names of the three isomers indicate which of the carbon-hydrogen units on
the benzene ring position of the molecule have been replaced by nitrogens,
called 1,2,3-triazine, 1,2,4-triazine, and 1,3,5-triazine respectively.
Symmetrical 1,3,5-triazine is the common. Triazines are prepared from
2-azidocyclopropene through thermal rearrangement (1,2,3-triazine), from
1,2-dicarbonyl compound with amidrazone by condensation reaction
(1,2,4-triazine) and from cyanic acid amide by trimerization (1,3,5-triazine).
Pyridine is the aromatic nitrogen heterocycle compound having only one nitrogen,
and diazines are with 2 nitrogen atoms and tetrazines are with 4 nitrogen atoms
on the benzene ring system. Triazines are weak base. Triazines have much weaker
resonance energy than benzene, so nucleophilic substitution is preferred than
electrophilic substitution. Triazines are basic structure of herbicides,
examples are amitole (CAS #: 61-82-5), atrazine (CAS #: 1912-24-9), cyanazine
(CAS #: 21725-46-2), simazine (CAS #: 122-34-9), trietazine (CAS #: 1912-26-1).
Large volume of triazines are used in the manufacture of resin modifiers such
as melamine and benzoguanamine. Melamine (1,3,5-Triazine-2,4,6-triamine) is
reacted with formaldehyde to from a very durable thermoset resin. Benzoguanamine
(2,4-Diamino-6-phenyl-1,3,5-triazine) is used to increase thermoset properties
of alkyd, acrylic and formaldehyde resins. Triazines are also useful as
chromophore groups in colorants and Chlorine attached in Triazine compounds
undergo nucleophilic substitution reactions well with with hydroxyl groups in
cellulose fibres. Some triazine family compounds are used in pharmaceutical
industry as coupling agent for the synthesis of peptide in solid phase as well
as solution and as side chain of antibiotics. Triazine compounds are used in
formulating bactericide and fungicide. They are used as preservatives in oil
field applications. They are used as disinfectant, industrial deodorant and
biocide in water treatment. They are used as a bleaching agents.
Tris(2-hydroxyethyl)
isocyanurate (THEIC) has the symmetrical triol structure and thus it can undergo
polymerization reactions. It is used as a monomer for the synthesis
of polyesters which are industrially used in a variety of coatings
(thermosetting paints, magnet wire enamels, electrical insulating
varnishes). Because of its trifunctionality, THEIC is used as a
precursor to crosslinking agents for rigid urethane foams and postforming
laminating resins.
THEIC is used as a stabilizer and heat resistant flame retardant of polymers.
One of the uses of such polymer is as exterior building material.
It is also used as an intermediate for the synthesis of dyes,
agrochemicals, pharmaceuticals and plasticizers. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
White crystalline powder |
| MELTING
POINT |
133
C - 137 C |
| HYDROXYL NUMBER |
630
- 650 (KOH mg/g) |
| ACIDITY |
1.0
max (KOH mg/g) |
| TRANSPORTATION |
| PACKING |
25kgs
in fiber drum |
| HAZARD
CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
|
Hazard
Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 26-37/39 |
|
GENERAL
DESCRIPTION
OF CYANURIC ACID
|
Cyanic acid
(the isomer of fulminic acid) is an unstable (explosive), poisonous, volatile,
clear liquid with the structure of H-O-C��N (the oxoacid formed from the
pseudohalogen cyanide), which is readily converted to
cyamelide and fulminic
acid. There is another isomeric cyanic acid with the
structure of H-N=C=O, called isocyanic acid. Cyanate group (and isocyanate group) can react with itself.
Cyanuric acid (also called pyrolithic acid), white monoclinic crystal with
the structure of [HOC(NCOH)2N], is the trimer
of cyanic acid. The
trimer
of isocyanic acid
is called biuret.
- Cyanic acid:
H-N=C=O
or H-O-C��N
- Fulminic acid: (H-C=N-O)
or H-C��N-O
- Isocyanic acid:
H-N=C=O
- Cyanuric acid:
HOC(NCOH)2N
- Biuret:
(NH2)CO)2 NH
Cyanic acid hydrolyses to ammonia and carbon dioxide in
water. The salts and esters of cyanic acid are cyanates. But esters of
normal cyanic acid are not known. The salts and esters of isocyanic acid are
isocyanates. The isocyanate group reacts with the hydroxyl functional group to form a urethane linkage.
Diisocyanates
(or polyisocyanates) are monomers for polyurethane production. Polyurethane is
made from a variety of diisocyanates in conjunction with polyether and polyester
polyols as co-reactants by addition polymerization which needs at least two
-N=C=O groups. Polyurethanes are widely used in the manufacture of flexible and
rigid foams, fibres, coatings, and elastomers. If isocyanate monomer is polymerized
with amine group,
polyurea is produced. Cyanates (or Isocyanates) are readily
reacts with various
form of amine (including ammonia, primary-, secondary-amines, amides and ureas)
and hydroxyl functional group. They are used in the synthesis
for the target molecules such as pharmaceuticals, pesticides, textile softener, lubricants and industrial
disinfectants. They can convert to polycyclic compounds such as hydantoins
and imidazolons. They are used as plastic additives and as heat treatment salt
formulations for metals.
|
